To My Quyen,
Nguyen Khanh Thuan, Nguyen Phuc Khanh, Nguyen Thanh Lam*
1. Introduction
Aspergillosis is an infectious, fungal disease caused by Aspergillus species, particularly Aspergillus fumigatus. Infection occurs by inhalation of spores and penetration through egg shell. The disease occurs in two forms, acute and chronic.
The acute form occurs by ingestion of large amount of spores, whereas, the chronic form affects birds with reduced immunity. Clinical signs include anorexia, emaciation, dyspnoea, gasping. Gross lesions includes white to yellowish granulomas of pea size. Microscopically peri-alveolar and pulmonary blood vessel congestion is seen. Diagnosis is based on history, clinical signs, necropsy findings, haemato-biochemical changes and culture of fungus. Treatment of aspergillosis is ineffective and prevention is the only way to control the disease. Best managemental practices like sanitation, avoiding wet litter or soil and dusty or moldy feeds, adequate ventilation, and disinfection of feed and waterlines should be carried out to prevent and control the disease (Kannoju et al., 2021).
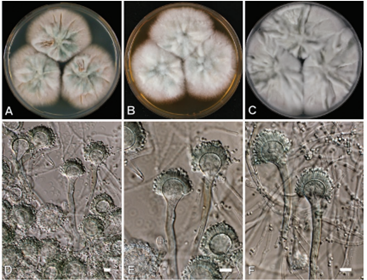
2. Aetiology
2.1 Bacterial characteristics
Conidiophores (400–800 μm long and 8–17 μm in diameter) of A. flavus are thick‐walled, rough (especially near the vesicle), and colorless. Vesicles, although more elongated when young, are globose to subglobose (20–45μm in diameter) with a row of cells (metulae) bearing phialides (biseriate) over the entire surface of the vesicle. Phialides bear chains of globose to subglobose, smooth to finely echinulate conidia with a diameter of 3–6μm (visibly larger than A. fumigatus spores). Some isolates are uniseriate, bearing a single layer of phialides directly on the vesicles (Swayne, 2013).
Aspergillus is a very large genus containing about 250 species, which are currently classified into seven subgenera that are in turn subdivided into several sections comprised of related species (Raper and Fennell, 1965; Geiser et al., 2007).
2.2 Classification
Aspergillosis in poultry is caused by a fungal species under the genus Aspergillus belonging to Kingdom: Fungi, Division: Ascomycota, Class: Eurotiomycetes, Order: Eurotiales, Family: Trichocomaceae. With various species namely- Aspergillus fumigatus, A. niger, A. flavus, A. terreus, and A. glaucus. But of all theses A. fumigatus is the most common cause of disease. These organisms are ubiquitous and saprophytic fungi which grow on organic matter in warm (>25°C) and also humid environment, and damaged egg in hatchery (Kannoju et al., 2021).
3. Epidemiology
3.1 Susceptible hosts
All birds are susceptible to aspergillosis. Inhalation of conidia or spores from contaminated feed, fecal material, soil and contamination of egg in ovo, infect the developing embryo. Higher susceptibility of birds to aspergillosis may be attributed to anatomic and physiologic characteristics of the avian respiratory system. The small non-expanding lungs and nine air sacs constitute a primary nidus for infection because the air (or conidia) reaches the caudal air sacs before it pass through those part of the lungs in which the gas exchange takes place (Nardoni et al., 2006).
3.2 Transmissions
Aspergillosis is not transmissible. Initial contamination of poultry farms may occur through the use of moldy litter, contaminated feedstuffs, or introduction of 1‐day‐old birds whose down has retained conidia in hatchery facilities. Further contamination may involve inappropriate bedding management. Constant animal movements under high stocking densities, litter refreshing, or deficient ventilation may contribute by generating a conidial aerosol. Fresh litter, especially if it has been wet previously and contaminated with A. fumigatus, is associated with outbreaks of aspergillosis. Dust appears to be an excellent carrier and reservoir for fungal spores (Dyar et al., 1984).
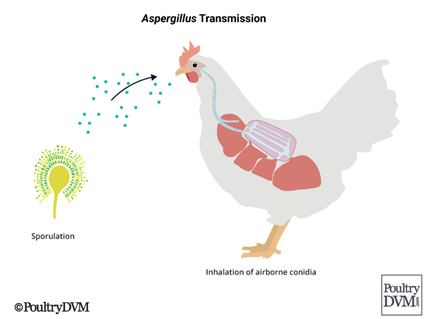
Therefore, distribution of very dry litter may generate large amounts of dust indoors. Chickens become infected with Aspergillus spores through exposure in their environment. Any disturbances which generate large amounts of dust particles, usually contain Aspergillus spores. If a chicken inhales significant quantities, it can cause disease (Figure 2). However, the inhaled spores may not cause disease right away, and instead remain dormant in the bird’s air sacs and lungs, until a stressful event or illness triggers Aspergillus to begin to cause damage (Lorenzoni, 2020).
3.3 Environment factors
Initial contamination of poultry farms may occur through use of a mouldy litter or introduction of one-day-old birds whose down has retained conidia in hatchery facilities. Further contamination may involve inappropriate bedding management, poor quality feedstuffs, or admission of outside air loaded in conidia (Martin et al., 2007). Organic substrates like litter, feed, and even feathers can easily fulfil nutrient requirements of A. fumigatus. Humidity and temperature conditions encountered in poultry farms promote the rapid growth of hyphae and efficient asexual multiplication resulting in a copious production of easily airborne hydrophobic conidia, which are subsequently dispersed and inhaled by the birds. Transfers of conidia between the putative bedding reservoir and indoor atmosphere are still poorly understood. Constant animal movements under high stocking densities, litter refreshing, or deficient ventilation may contribute to generate a conidial aerosol. A short-time exposure to heavily contaminated wood shavings induced an experimental pulmonary aspergillosis in chickens and turkeys. Birds inhale the air and contact litter with continual exposure to the conidia (Arné et al., 2011).
4. Pathogenesis
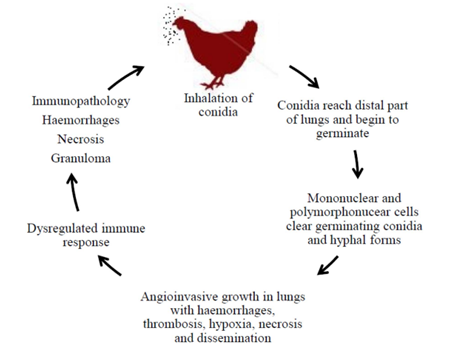
Sequential necropsies at various times after inoculation allow observations on the pathogenesis of the disease and the kinetics of internal lesions development. Immediately after aerosol exposure and up to 24 h later, A. fumigatus conidia were detected in circulating blood of 3-week-old turkeys. In contrast with mycological cultures of lung tissue, the proportion of Aspergillus-positive cultures from liver and brain decreased with time. Numerous conidia could be observed in many macrophages harvested by lung lavages. Therefore, brain or eye lesions due to A. fumigatus may follow rapid haematogenous dissemination of conidia mediated by macrophage migration from the respiratory system (Richard et al., 1984). Virulence represents the ability of a pathogen to invade the host, overcome its natural defences, and proliferate subsequently in the organism (Figure 3).
Air sac membranes were slightly opaque 24 h after air sac inoculation and scattered with miliary white foci. As fulminant airsacculitis evolved, the severity of macroscopic lesions increased rapidly, with progressive thickening, vascularisation, and opacification. Concurrently, 1–5 mm granulomas developed and tended to coalesce in plaques. Early lesions in lungs consisted of marginal oedema, progressive consolidation, and the formation of small white nodules (Faublée, 1975).
In the first hours following infectious challenge, clear oedema, extensive epithelial alterations, and massive infiltration of heterophils, macrophages, and lesser lymphocytes occurred in both lungs and air sacs. Scarce swollen and germinating conidia were observed in inflammatory exudates, in areas of necrosis and aggregates of epithelial macrophages or multinucleated giant cells. A. fumigatus seemed to infect the air sac membrane interstitium rather than its surface leading to a severe airsacculitis within 24 h. Granulomas with heterophilic or necrotic centres and fungal elements were reported as soon as 24 h after air sac inoculation in 9- and 19-week-old turkeys, but not in one-day-old turkeys, where diffuse inflammation predominated till 48 h. Chronologically, multifocal inflammatory response was gained in cellularity with marked mixed cellular infiltrates and granulomatous reaction in respiratory tissues. Circumscribed pyogranulomas developed with central eosinophilic necrosis restricting short hyphae and conidia surrounded by intact heterophils, epithelioid, and foreign body giant cells. Beyond 6 days after exposure, inflammatory and necrotic foci seemed to regress in surviving birds exhibiting well-organized granulomas encapsulated by a thick layer of fibrous tissue. Destruction of A. fumigatus occurred as attested by fungal elements observed in multinucleate giant cells and the regular diminution of positive cultures from tested organs (Kunkle and Rimler, 1996).
5. Clinical signs and pathology
5.1 Clinical signs
Acute aspergillosis may include a variety of nonspecific clinical signs: anorexia, lethargy, ruffled feathers, respiratory signs, polydipsia, polyuria, stunting, or sudden death. In chicks, contaminated in ovo or during hatching, the disease, commonly known as brooder pneumonia, is highly fatal in the first ten days of life and results in a major respiratory distress .Two outbreaks of omphalitis where the primary cause was A. fumigatus have been investigated in young turkeys. In poultry farms, mortality rate may rise slightly or increases suddenly, peaks during a few days, and then returns to initial state. Respiratory signs include dyspnoea, gasping, hyperpnoea with panting, nonproductive coughing, wheezing, cyanosis, and sometimes nasal discharge (Singh et al., 2009).

In the chronic form, dyspnoea, depression, dehydration, and emaciation are described. Nervous system involvement causes ataxia, tremor, opisthotonos, lateral recumbency, torticollis, seizures, convulsions, lameness, and hind limb paresis. Occurrence of nervous and ophthalmic troubles one week after an acute episode of aspergillosis has been reported in a turkey flock. Cloudiness of the eye with severe conjunctivitis and turbid discharge were associated with paralysis in broiler breeders.
A. fumigatus can colonize skin and surgical wounds as observed in caponized cockerels and induce necrotic granulomatous dermatitis or even systemic aspergillosis (Arné et al., 2011).
5.2 Pathology
Macroscopic lesions
Lesions of uncomplicated pulmonary aspergillosis evolve over several days and diminish in a few weeks. The primary location of lesions is the air sacs, pleura, and lungs although eye, esophagus, proventriculus, gizzard, small intestine, liver, kidney, spleen, skin, trachea, peritoneum, brain, eye, muscle, heart, bones, articulation, and yolk sac may be involved. Acute lesions of experimental aspergillosis in turkeys rapidly progress in severity. White miliary foci were present on air sac membranes assoon as 24 hours’ post‐air sac inoculation in 9–19 week‐old turkeys but not before 48 hours in 5‐day‐old poults. Lung lesions consisted of congestion, parenchyma consolidation, and straw‐colored gelatinous subpleural edema. Air sacs became progressively thicker and opaque with focal granulomas that increased in size and changed shape from raised domes (1mm) to flat or umbilicated plaques (2–5mm) that tended to coalesce. Extensive white discoloration of lungs and granulomatous pneumonia were evident by 72 hours after intra‐air sac infection with A. fumigatus (Kunkle and Rimler, 1996).
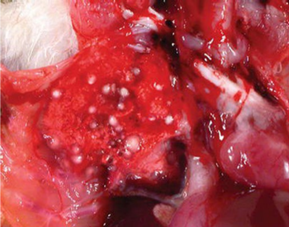
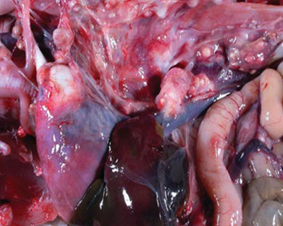
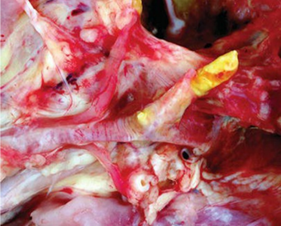
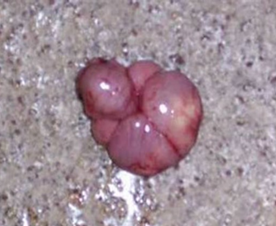
Lung lesions in experimental aerosol infection of turkey poults consisted of small white caseous nodules (approximately 1–3mm in diameter) scattered throughout lung tissue (Figure 5), usually accompanied by similar sized caseous plaques on thickened air sac membranes (Figure 6). Occasionally, red‐tinged ascites was present. In an outbreak of aspergillosis in chicks, yellow foci were not found but lungs were a diffuse gray‐yellow.
In advanced cases of aspergillosis, air exposure can cause the organism to sporulate on the surface of caseous lesions and on the walls of thickened air sacs, producing visible green‐gray mold growth.
Caseous, gelatinous, or less commonly mucohetero-philic exudate may be present in the syrinx of infected birds (Figure 7). Localized tracheal aspergillosis caused by A. flavus has been characterized by grossly visible yellow caseous plaques that adhered to the mucosal surface, occasionally occluding the trachea and reddened tracheal walls (Swayne, 2013).
Microscopic lesions
Based on histopathological differences, a deep nodular form and a superficial diffuse form of aspergillosis can be distinguished. A well‐organized granulomatous reaction develops in nonaerated parenchyma whereas a superficial diffuse form, containing fungal elements and a nonen-capsulated pyogranulomatous reaction, predominates in serosae and lungs. Organized granulomas are clearly encapsulated by an outer thick fibrous layer whereas pyogranulomas lack clear borders. In a study of acute pulmonary aspergillosis in turkey poults, granulomatous airsacculitis and pleuritis were seen as early as 24 hours after intra‐air sac inoculation with A. fumigatus. Air sac membranes were thickened up to 100‐fold by massive infiltrates of heterophils, multinucleate giant cells, and other leukocytes. Germinating conidia were seen in the membrane interstitium, and lymphohistiocytic perivasculitis was discernible in less severely affected areas. Granulomas had centers composed of necrotic cellular debris and heterophils with a peripheral palisade of epithelioid macrophages and aggregates of lymphocytes (Figure 9). Examination of heterophilic granulomas stained with GMS stain revealed large numbers of germinating conidia centrally and hyphae extending peripherally through the layer of macrophages lymphohistiocytic or granulomatous pleuritis and pneumonia with edema and hemorrhage in the initial 48 hours, but had progressed to extensive effacement of parenchymal architecture because of necrosis, hemorrhage, and massive infiltrates of leukocytes by 72 hours (Swayne, 2013).
In 2 alternative models of acute aspergillosis in turkeys, from 20% to 95% of viable lung parenchyma were progressively replaced by multifocal‐to‐coalescing necrogranulomas. Epithelioid macrophages admixed with multinucleate giant cells were arranged in sheets. Intact and degenerate heterophils predominated in areas of necrosis. Septate hyphae were mostly localized to areas of necrosis and aggregates of multinucleate giant cells. Vascular invasion with thrombosis is often seen in severe cases. By 120 hours, residual fungal elements were restricted to the cytoplasm of giant cells highlighting the ability of some birds to control the development of the infection through an effective immune response (Swayne, 2013).
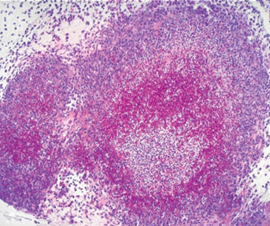
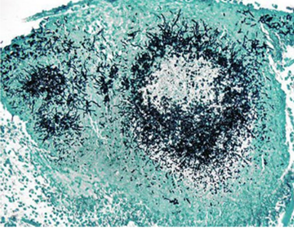
Nonviable A. fumigatus conidia produced a transient airsacculitis and pneumonia characterized by edema and infiltrates of heterophils and macrophages (Kunkle and Rimler, 1996). Multinucleate giant cells were not present in these lesions, in contrast to active infections with Aspergillus, in which both epithelioid macrophages and multinucleate giant cells were prominent features. In a study of subacute and chronic phases of aspergillosis, examination of lung tissues from turkey poults revealed no differences in histopathologic lesions caused by A. fumigatus or A. flavus. Lesions seen in the first 2 weeks of the study were characterized by focal accumulations of lymphocytes, some macrophages, and a few giant cells. Later, lesions consisted of granulomas with a central area of necrosis containing heterophils surrounded by macrophages, giant cells, lymphocytes, and some fibrous tissue. By 8 weeks postexposure, surviving poults had mature fibrous granulomatous lesions consisting of necrotic centers surrounded by giant cells and a thick layer of fibrocytes and collagen containing a few scattered heterophils. Using Gridley’s fungal stain, the organisms could be seen within the necrotic areas of the lesions. Areas of conidial production were seen in tissue sections of the well‐oxygenated bronchi, bronchioles, and air sacs upon which plaques became velvety and change color to shades of green, olive, brown, or black depending on the fungal species involved. The presence of a fibrous capsule at the periphery of the granulomas and of multinucleated giant cells associated with focal lymphoid infiltration in the pulmonary parenchyma might be an important sign of the chronic form of the disease (Beytut, 2007).
6. Diagnosis
6.1 Isolation and identification of causative agent
Diagnosis of aspergillosis, particularly in chronic cases, remains a challenge. It relies on a combination of clinical signs, epidemiological considerations, and eventually culture and cytology on tracheal or air sac lavage in poultry. Necropsy may reveal characteristic caseous nodules in the lungs or plaques in the air sacs of affected birds. Aerated tissues are sometimes lined by a grey‐green velvet when fungal sporulation occurs. Definitive diagnosis requires the identification of Aspergillus isolated by culture or by the detection of the organism during histological examination on biopsy specimens (Swayne, 2013).
6.2 Serologic tests
Serologic tests are of limited value because of the non-specific nature of the fungal antigens. Agar gel precipitin has been reported in comparisons of A. fumigatus and A. flavus infections in turkey poults. Although most of the A. fumigatus‐infected poults were positive for precipitating antibodies, poults infected with A. flavus were not. Antibody response, as measured by ELISA and agar gel immunodiffusion, was erratic, although most poults with high antibody scores had marked lesions and low weight. Additionally, a direct ELISA technique has been used in turkeys with a correlation occurring between exposure level and ELISA titer. Perhaps use of serologic methods to identify poults with aspergillosis for culling would be advantageous because there is no legal or effective therapy for treating positive birds (Swayne, 2013).
6.3 Haematoxylin and eosin staining
The tissue samples (lungs, trachea, pharynx and thoracic air sacs as well as other organs) fixed in 10% neutral buffered formalin are processed and embedded in paraffin blocks and stain with haematoxylin and eosin (HE) method. Aspergillus hyphae stained poorly in H and E stained sections. Differential stains such as Periodic acid-Schiff (PAS), Bauer’s and Gridley’s stains differentiate and easily identify the hyphae and mycelia. Special stains for fungus Grocott’s and Gomori Methanamine Silver stain should be employed to detect the presence of fungal hyphae. For proper identification of the species, the pathogenic organism must be isolated by culturing it on differential media. Small pieces of lesions aseptically removed are placed onto plates or slants containing malt agar, Sabouraud’s glucose agar or antibiotics and incubated at 37°C for 24 hours. Species of Aspergillus can be identified by observing the characteristic conidial head and colony (Fowler and Miller, 2007).
6.4 Differential diagnosis
The differential diagnosis includes infectious bronchitis (dyspnea), newcastle disease (watery greenish diarrhea), infectious larngeo tracheaties (gasping, cough and extension of the neck during inspiration), dactylaria infection (nervous sign) and nutritional encephalomalacia (Baker, 1998).
7. Treatment
Treatment for aspergillosis is not effective because the drug used does not reach the fungus that is walled off by the bird’s inflammatory response and therefore, isolated from the bloodstream. This disease has a poor prognosis when the infection in the tissue is extensive and only systemic drugs are used. The best treatment results if the granulomatuos lesion is dried and topical treatment in conjunction with systematic therapy is given. Treatment of aspergillosis involves the use of one or more systemic antifungal agents. Drugs which are commonly used include itraconazole, ketoconazole, clotrimazole, miconazole, fluconazole and Amphotercin B. From these drugs, itraconazole is a choice of treatment of the disease (Sultana et al., 2015).
8. Control and prevention
Although numerous antifungal protocols have been proposed to cure birds with aspergillosis, treatment of the disease in poultry farms is virtually impossible. No vaccine is available. Specific biosecurity measures against Aspergillus contamination rely primarily on prevention. Dust and mouldy litter or feed should be avoided. A good litter management combined with daily assessment of its quality throughout the lifetime of the flock is the key to prevention of the disease. Bed, like feeders, should be kept dry, nondusty, and clean in order to limit fungal development.
Control of relative humidity through appropriate ventilation should be verified to prevent wet litter. Sporadic or repeated antifungal treatment may be useful in order to control environmental contamination. Spraying of fungistatic agents like thiabendazole, nystatin, or copper sulphate contributed to decreased fungal contamination of beddings. Enilconazole may be sprayed, fogged, or nebulised to decontaminate surfaces or indoor volume. Finally, effects of stressors like beak trimming and high stocking densities should be minimized (Swayne, 2013).
9. References
Arné, P., Thierry, S., Wang, D., Deville, M., Le Loc’h, G., Desoutter, A., Féménia, F., Nieguitsila, A., Huang, W., Chermette, R., Guillot, J., 2011. Aspergillus fumigatus in Poultry. International journal of microbiology 2011, 746356.
Baker, J.R., 1998. Avian Medicine and Surgery. Avian Pathology 27, 107.
Beytut, E., 2007. Immunohistochemical diagnosis of aspergillosis in adult turkeys. Turkish Journal of Veterinary & Animal Sciences 31, 99-104.
Dyar, P., Fletcher, O., Page, R., 1984. Aspergillosis in turkeys associated with use of contaminated litter. Avian Diseases, 250-255.
Faublée, V., 1975. Rôle pathogéne expérimental d’Aspergillus fumigatus chez le poulet.
Fowler, M.E., Miller, E.R., 2007. Zoo and Wild Animal Medicine Current Therapy-E-Book. Elsevier Health Sciences.
Geiser, D., Klich, M., Frisvad, J.C., Peterson, S., Varga, J., Samson, R.A., 2007. The current status of species recognition and identification in Aspergillus. Studies in mycology 59, 1-10.
Kannoju, A., Veldi, P., Kumar, V., 2021. An overview of aspergillosis in poultry: A review. Journal of Entomology and Zoology Studies 9, 685-688.
Kunkle, R., Rimler, R., 1996. Pathology of acute aspergillosis in turkeys. Avian Diseases, 875-886.
Lorenzoni, G., 2020. Aspergillosis in Poultry, PoultryDVM, Penn State Extension
Martin, M.P., Pecelunas Bouck, K., Helm, J., Dykstra, M.J., Wages, D.P., Barnes, H.J., 2007. Disseminated Aspergillus flavus infection in broiler breeder pullets. Avian Diseases 51, 626-631.
Munir, M., Rehman, Z., Shah, M., Umar, S., 2017. Interactions of Aspergillus fumigatus with the respiratory system in poultry. World’s Poultry Science Journal 73, 321-336.
Nardoni, S., Ceccherelli, R., Rossi, G., Mancianti, F., 2006. Aspergillosis in Larus cachinnans micaellis: survey of eight cases. Mycopathologia 161, 317-321.
Raper, K.B., Fennell, D.I., 1965. The genus Aspergillus. The genus Aspergillus.
Richard, J., Thurston, J., Peden, W., Pinello, C., 1984. Recent studies on aspergillosis in turkey poults. Mycopathologia 87, 3-11.
Singh, S., Borah, M., Sharma, D., Joshi, G., Gogoi, R., 2009. Aspergillosis in turkey poults. Indian Journal of Veterinary Pathology 33, 220-221.
Sultana, S., Rashid, S., Islam, M., Ali, M., Islam, M., Azam, M., 2015. Pathological investigation of avian aspergillosis in commercial broiler chicken at Chittagong district. International Journal of Innovation and Applied Studies 10, 366.
Swayne, D.E., 2013. Diseases of poultry. John Wiley & Sons.


