To My Quyen,
Nguyen Khanh Thuan, Nguyen Phuc Khanh, Nguyen Thanh Lam*
1. Introduction
Infectious coryza is an acute respiratory disease of chickens caused by the bacterium Avibacterium paragallinarum. It is characterized by decreased activity, nasal discharge, sneezing, and facial swelling that occurs worldwide. The disease apparently affects only chickens; reports in quail and pheasants likely describe a similar disease caused by a different bacterium. Diagnosis is based on PCR assay, bacterial culture, or production of typical clinical signs in susceptible chickens following inoculation with nasal exudate from an infected bird. In lower- and middle-income countries, the disease often is seen in very young chicks (e.g., 3 weeks old). Inadequate biosecurity practices and environmental factors may contribute. Infectious coryza is not a zoonotic disease, and thus lacks public health importance (Soriano-Vargas, 2022).
2. Aetiology
2.1 Bacterial characteristics
The causative bacterium of infectious coryza is Avibacterium paragallinarum, a gram-negative, pleomorphic, nonmotile, catalase-negative, microaerophilic rod that requires nicotinamide adenine dinucleotide (V-factor) for culture. When cultured on blood agar with a staphylococcal nurse colony that excretes the V-factor, the satellite colonies appear as dewdrop shapes, growing adjacent to the nurse colony. V-factor–independent A. paragallinarum have been reported in South Africa and Mexico. The most commonly used serotyping scheme is the Page scheme, which groups A. paragallinarum isolates into three serovars (A, B, and C) that correlate with immunotype specificity (Soriano-Vargas, 2022).
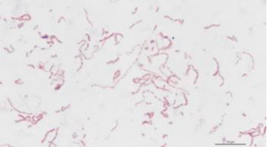

2.2 Classification
Page., 1962 classified this bacterium into three serogroups A, B, and C, according to the plate agglutination test (Page, 1962). Later, these schemes were amplified to recognize nine Kume serovars: A-1, A-2, A-3, A-4, B-1, C-1, C-2, C-3, and C-4 using hemagglutination inhibition tests. Nevertheless, these schemes might need to be revised and updated as many B strains have been described as different.
The distribution of the three Page serogroups differs from country to country. Nevertheless, not all the countries report the distribution of the different serogroups. The three Page serogroups were reported in Argentina, Brazil, China, Ecuador, Egypt, Germany, India, Indonesia, Peru, Philippines, Mexico, South Africa, Spain, and the USA. Furthermore, in Australia, Israel, and Japan, only serogroups A and C have been reported, while in Zimbabwe, only serogroups B and C are known. In Malaysia, only serogroup A, in Panama and Thailand only serogroup B, while in Taiwan only serogroup C were described. Moreover, some serogroup B strains were reported in Argentina, Ecuador, Peru, the USA, and Zimbabwe as variants (Bvar), and, as above-mentioned, might represent a new immunotype (Caballero-Garcia et al., 2022).
3. Epidemiology
3.1 Susceptible hosts
Chronically ill or healthy carrier birds are the reservoir of infection for A. paragallinarum. Chickens of all ages are susceptible; however, susceptibility increases with age. The incubation period is 1–3 days with a typical disease duration of 2–3 weeks. Duration of illness may be longer in the presence of concurrent diseases such as mycoplasmosis (Soriano-Vargas, 2022).
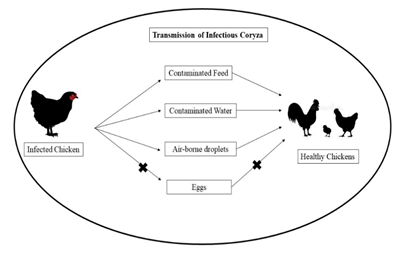
3.2 Transmissions
Infected flocks are a constant threat to uninfected flocks. Transmission is by direct contact, airborne droplets, and contamination of drinking water (Figure 3). Transmission does not occur via eggs. “All-in/all-out” management has essentially eradicated infectious coryza from many commercial poultry operations in the US. Such programs move all animals of the same age at the same time. This flow allows birds in the same enclosures to be exposed to any pathogen(s) at the same time. Facilities are disinfected before new next group of new animals are added. Commercial farms without such flow and multiple-age flocks may continue to see outbreaks of the disease. Molecular techniques such as restriction endonuclease analysis and ribotyping have been used to trace outbreaks of infectious coryza (Cigoy et al., 2016).
4. Clinical signs, gross leisons and histopathological study
4.1 Clinical signs
The incubation period (IP) for disease is 1-3 days and typical duration of disease persists for 2-3 weeks. However, the period varies in experimental inoculation of birds with live Aribacterium paragallinarum or the infectious exudates through different routes. Through intranasal inoculation, IP was 24 hour, nasal inoculation 48 hours, 3 days for birds in cage, 4 days for infected drinking water contact and 6-14 days for airborne transmission of bacteria. Disease has low mortality and high morbidity (Poudel, 2022).

Clinical signal occur in two forms: mild form usually occurs in young chickens and severe form in adults and older birds (Figure 4). In mild form of coryza, signs may be listlessness, depression, a serons nasal discharge and mild facial swelling. The affected birds reduced feed and water consumptions. In severe form, there will be swelling of the infraorbital sinus with sneezing, serous, mucold or suppurative nasal discharge. The facial edema prevents eyelids from fully opening leading to conjunctivitis and epiphora. In males, cedematous swelling in intermandibular spaces and wattles is seen. Birds may have varying degrees of rales due to poeumonia and air sacculitis. In some commercial broilers, uncommon signs of otitis and meningoencephalocele were observed. There is massive reduction in egg production in layers and delayed egg laying in young pullets. Moreover, presence of secondary pathogens especially, mycoplasmas and virus, and stress factors can result in overall disease complexity (Poudel, 2022).
4.2 Gross study
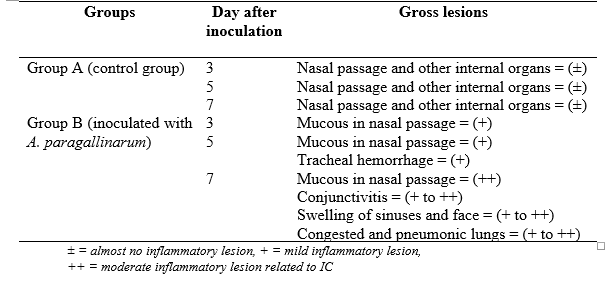
A study has conducted to reasearch the gross leisons of chickens that are infected with A. paragallinarum. All the internal organs including nasal passage were examined and gross lesions were recorded. The inflammatory lesions of different organs (congestion, hemorrhage, swelling, mucus, etc.) were graded as ± = almost absence of lesion, + = mild lesion, ++ = moderate lesion and +++ = severe lesion.

Table 1: Gross lesions in different organs in infectious coryza (Ali et al., 2013)
4.3 Histopathological study
All of the microscopic lesions have been presented in Table 2 and illustrated in Figure 7-10. The microscopic lesions of the chicks of this group were acanthosis and congested blood vessels of nasal passage and pneumonic lesion of lung which were progressively prominent on day 7 of bacterial inoculation. All of these studies have very close agreements with studies of Sawata et al. (1985) and Droual et al. (1990) who studied the microscopic changes due to infectious coryza (Droual et al., 1990; Sawata et al., 1985).
Table 2: Results of histopathological studies of group B inoculated with A. paragallinarum (Ali et al., 2013)


Figure 8: Nasal passage of group B (inoculated with A. paragallinarum) on day 7 of post inoculation showing acanthosis (arrow), parakeratosis and/mucous (H & E stain, X 333) (Ali et al., 2013)

Figure 10: Lung of group B (inoculated with A. paragallinarum) on day 7 of post inoculation showing severe pneumonic lesion (+++) (H & E stain, X 333) (Ali et al., 2013)
5. Diagnosis
5.1 Isolation and identification of causative agent
Although A. paragallinarum is considered to be a fastidious organism, it is not difficult to isolate, requiring simple media and procedures. Specimens should be taken from 2 or 3 chickens in the acute stage of the disease (1–7 days’ incubation). The skin under the eyes is seared with a hot iron spatula and an incision made into the sinus cavity with sterile scissors. The swab is streaked on a blood agar plate, which is then crosss treaked with a Staphylococcus culture and incubated at 37 °C in a large screw‐cap jar in which a candle is allowed to burn out (Blackall and Soriano‐Vargas, 2020).
5.2 Serology
There is no totally suitable serological test for the diagnosis of IC. However, despite this absence of a “perfect” test, serological results are often useful for retrospective/ epidemiological studies in the local area.
The simple HI is based on whole bacterial cells of Page serovar A A. paragallinarum and fresh chicken erythrocytes. Although simple to perform, this HI test can only detect antibodies to serovar A. The test has been widely used to both detect infected as well as vaccinated chickens . A variation of this test (whole bacterial cells and glutaraldehyde‐fixed chicken erythrocytes) has been shown to detect antibodies caused by all 9 Kume serovars in vaccinated chickens.
The extracted HI test is based on KSCN‐extracted and sonicated cells of A. paragallinarum and glutaraldehyde‐fixed chicken erythrocytes. This extracted HI test has mainly been validated for the detection of antibodies to Page serovar C organisms. The test has been shown to be capable of detecting a serovar‐specific antibody response in Page serovar C vaccinated chickens. A major weakness with this assay is that, in chickens infected with serovar C, the majority of the birds remain seronegative (Blackall and Soriano‐Vargas, 2020).
5.3 PCR testing
The polymerase chain reaction (PCR) was standardized for the diagnosis of infectious coryza by using infectious coryza Killed vaccine, ventri biologicals, Pune as source of DNA of A. paragallinarum (Muhammad and Sreedevi, 2015).
6. Treatment
Early treatment is considered as really important step, therefore; immediate administration of medication via drinking water is recommended until medicated feed is available. Erythromycin and oxytetracycline are usually effective. Additionally, several newer-generation antimicrobials (e.g., fluoroquinolones, macrolides) are active against infectious coryza. Various sulfonamides, including trimethoprim-sulfamethoxazole, and other drug combinations have been successful for treatment. Antimicrobial use in chickens is subject to national regulations that vary from country to country, and use and efficacy must be reviewed in light of relevant laws. In more severe outbreaks, although treatment may result in improvement, the disease may recur when medication is discontinued. Preventive medication may be combined with a vaccination program if started pullets are to be reared or housed on infected premises (Soriano-Vargas, 2022).
Early treatment with supportive care is highly important for alleviating the severity and course of infection of infectious coryza. Various chemotherapeutics and antibiotics can be administered through feed or drinking water for successful treatment of infectious coryza (Cigoy et al., 2016). Infected birds respond to treatment however, disease may relapse when treatment is discontinued and the carrier state of infected birds will persist for long time. So, at least for a week enrofloxacin at 10 mg/kg body weight or amoxicillin at 20 mg/kg body weight should be given. Sulfonamides, in combination with other drugs like trimethoprim- sulfamethoxazole or sulphachloropiridazine-trimetroprim via drinking water also give good results. Sulfa drugs cause a temporary drop in egg production and overdose may lead to renal toxicity. In case of secondary infection, especially with mycoplasma spp., enrofloxacin (20-25 mg/kg) or kanamycin (30 mg/kg) with gentamicin (5-8 mg/kg) or tylosin tartrate (30 mg/kg) with streptomycin at 100 mg/kg should be given. Use of streptomycin can cause severe stress in the young chicken for about one day. Erythromycin and oxytetracycline are commonly used and effective two antibiotics for treatment of disease (Soriano-Vargas, 2022).
7. Control and prevention
Prevention is the only sound method of control for infectious coryza. All-in/all-out flow of animals as part of sound farm management and biosecurity practices are important disease prevention measures. Replacement chickens should be raised on the same farm or obtained from clean flocks. If replacement pullets are to be placed on a farm that has a history of infectious coryza, bacterins/vaccines are available to help prevent and control the disease. USDA-licensed commercially produced bacterins are available, and bacterins also are produced within states for intrastate use. Bacterins/vaccines also are produced in many other countries. Because serovars A, B, and C are not cross-protective, it is essential that bacterins contain the serovars present in the target population.
Vaccination on individual farms should be completed ~4 weeks before infectious coryza outbreaks typically occur. Antibodies detected by the hemagglutination-inhibition test after bacterin administration do not necessarily correlate with protective immunity. Controlled exposure to live organisms also has been used to produce protective immunity in layers in endemic areas (Soriano-Vargas, 2022).
8. References
Akter, S., Ali, M., Das, P., Hossain, M.J.J.o.t.B.A.U., 2013. Isolation and identification of Avibacterium paragallinarum, the causal agent of infectious coryza (IC) from layer chickens in Bangladesh. 11, 87-96.
Ali, M., Hossain, M., Akter, S., Khan, M., Hossain, M.J.T.A., 2013. Pathogenesis of infectious coryza in chickens (Gallus gallus) by Avibacterium paragallinarumn isolate of Bangladesh. 11, 39-46.
Blackall, P.J., Soriano‐Vargas, E.J.D.o.p., 2020. Infectious coryza and related bacterial infections. 890-906.
Caballero-Garcia, M., Mendoza-Espinoza, A., Ascanio, S., Chero, P., Rojas, R., Huberman, Y.D., 2022. Pathogenicity of Avibacterium paragallinarum Strains from Peru and the Selection of Candidate Strains for an Inactivated Vaccine. Vaccines 10.
Cigoy, M., Huberman, Y., Terzolo, H.J.R.J., , from Engromix, Poultry Industry, Technical Article website: https://en. engormix. com/poultry-industry/articles/infectious-coryza-t39047. htm, 2016. Infectious coryza.
Droual, R., Bickford, A., Charlton, B., Cooper, G., Channing, S.J.A.d., 1990. Infectious coryza in meat chickens in the San Joaquin Valley of California. 1009-1016.
Muhammad, T.M., Sreedevi, B., 2015. Detection of Avibacterium paragallinarum by Polymerase chain reaction from outbreaks of Infectious coryza of poultry in Andhra Pradesh. Veterinary world 8, 103-108.
Page, L.J.A.J.V.R., 1962. Haemophilus infections in chickens. 1. Characteristics of 12 Haemophilus isolates recovered from diseased chickens. 23, 85-95.
Poudel, U., 2022. Infectious Coryza in poultry: A mini-review.
Sawata, A., Nakai, T., Kume, K., Yoshikawa, H., Yoshikawa, T.J.A.j.o.v.r., 1985. Intranasal inoculation of chickens with encapsulated or nonencapsulated variants of Haemophilus paragallinarum: electron microscopic evaluation of the nasal mucosa. 46, 2346-2353.
Soriano-Vargas, E., 2022. Infectious Coryza in Chickens. MSD Manual. Veterinary Manual.
Talha, A., Hossain, M., Chowdhury, E., Bari, A., Islam, M., Das, P.J.T.B.V., 2001. Poultry diseases occurring in Mymensingh district of Bangladesh. 18, 20-23.


